H2S04, commonly known as sulfuric acid, is a chemical powerhouse that plays a vital role in various industrial processes and scientific research. This colorless, odorless liquid is one of the most widely produced chemicals globally and is known for its highly corrosive nature. As we delve deeper into this fascinating compound, we will explore its applications, properties, and the critical role it plays in our daily lives.
From batteries to fertilizers, H2S04 is an essential ingredient in numerous products that we often take for granted. Its ability to act as a dehydrating agent and strong acid makes it a versatile solution in laboratories and factories alike. In this article, we will discuss the chemical structure of H2S04, its historical background, and the safety measures necessary when handling this powerful acid.
As we embark on this journey to uncover the intricacies of H2S04, we invite you to consider the importance of understanding this chemical compound. Knowing how to safely work with and utilize sulfuric acid can significantly impact both industrial efficiency and environmental safety. With this in mind, let’s dive into the world of H2S04!
What is H2S04 and Its Chemical Structure?
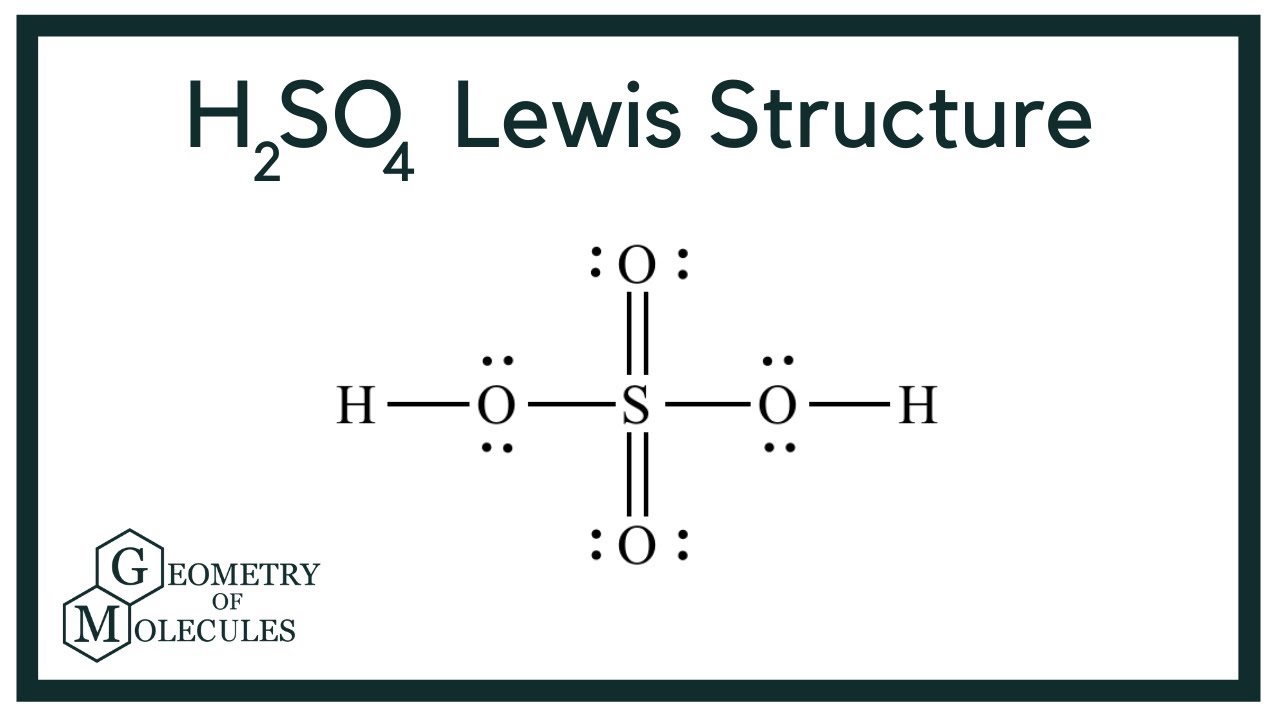
H2S04, or sulfuric acid, has a molecular formula that consists of two hydrogen atoms, one sulfur atom, and four oxygen atoms. The structure of H2S04 can be represented as follows:
- Two hydrogen (H) atoms
- One sulfur (S) atom
- Four oxygen (O) atoms
This composition allows sulfuric acid to exhibit unique properties, such as its high boiling point and excellent solubility in water, making it a crucial component in many chemical reactions.
What are the Uses of H2S04 in Industry?
H2S04 is indispensable in various industries. Here are some notable applications:
- Fertilizer Production: Sulfuric acid is a key ingredient in the manufacturing of phosphate fertilizers.
- Battery Manufacturing: It is used as an electrolyte in lead-acid batteries.
- Petroleum Refining: H2S04 is employed in refining processes to remove impurities.
- Wastewater Treatment: It helps neutralize alkaline waste before disposal.
How is H2S04 Produced?
The most common method for producing H2S04 is the Contact Process, which involves the following steps:
- Sulfur Dioxide Generation: Sulfur is burned in the presence of oxygen to produce sulfur dioxide (SO2).
- Oxidation: The sulfur dioxide is then oxidized to sulfur trioxide (SO3) using a catalyst.
- Absorption: The sulfur trioxide is absorbed in water or oleum to produce sulfuric acid.
What are the Safety Precautions When Handling H2S04?
Due to its corrosive nature, handling H2S04 requires stringent safety measures. Here are some essential precautions:
- Wear appropriate personal protective equipment (PPE), including gloves and goggles.
- Work in a well-ventilated area or fume hood to avoid inhaling fumes.
- Always add acid to water, never the other way around, to prevent violent reactions.
- Have neutralizing agents and emergency equipment readily available.
What are the Environmental Impacts of H2S04?
While H2S04 is essential for many processes, it can have detrimental effects on the environment if not managed properly:
- Acid Rain: Sulfur dioxide emissions can lead to the formation of acid rain, harming ecosystems.
- Soil Degradation: Improper disposal can lead to soil acidification, negatively affecting plant growth.
What are the Historical Uses of H2S04?
Historically, sulfuric acid has been a cornerstone of various scientific advancements. It was first discovered in the 8th century by the Arab chemist Jabir ibn Hayyan. Over the centuries, it has been used in:
- Alchemy and early chemistry experiments.
- The production of gunpowder.
- Textile manufacturing processes.
What is the Future of H2S04 in Technology?
As technology advances, the role of H2S04 continues to evolve. Innovations in battery technology, such as electric vehicles, rely heavily on sulfuric acid for energy storage solutions. Furthermore, researchers are exploring environmentally friendly methods for sulfuric acid production, which may significantly reduce its ecological footprint.
Conclusion: Why Understanding H2S04 is Crucial?
In summary, H2S04 is more than just a chemical compound; it is a vital resource that drives numerous industries and scientific innovations. Understanding its properties, applications, and safe handling practices is essential for anyone working with this powerful acid. As we move towards a more sustainable future, the significance of H2S04 will undoubtedly continue to grow, making it imperative for us to grasp its potential and implications thoroughly.
Article Recommendations
- Top Klecko Jets Deals Reviews
- Mitch Mcconnell Stuttering Video Shocking Footage
- Travis S Taylors Wife Unveiling The Mystery