The BCl3 Lewis structure is a crucial aspect of understanding the chemical behavior of boron trichloride. This compound is significant in various chemical processes and applications, making it essential for students and professionals alike to grasp its structure and bonding. By visualizing the Lewis structure, one can predict molecular geometry, reactivity, and polarity, which are vital for applications in fields like chemistry, material science, and engineering.
In this article, we will delve into the details of the BCl3 Lewis structure, exploring its formation, characteristics, and the implications of its molecular geometry. Understanding the arrangement of valence electrons and how they contribute to bond formation will provide insights into the stability and reactivity of boron trichloride. Whether you're a chemistry student preparing for an exam or simply curious about molecular structures, this guide will equip you with the necessary knowledge.
Join us as we explore the BCl3 Lewis structure, answering common questions and providing clear explanations. By the end of this article, you'll have a deeper understanding of this fascinating compound and its significance in the world of chemistry.
What is the BCl3 Lewis Structure?
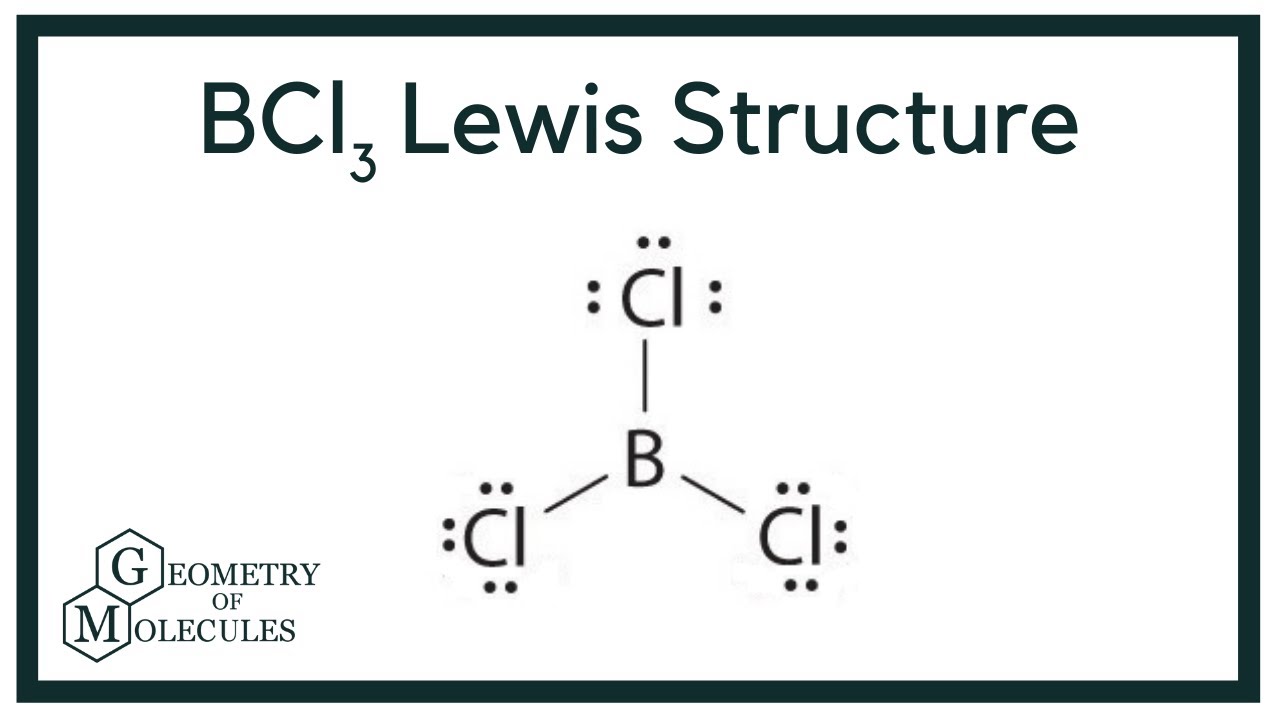
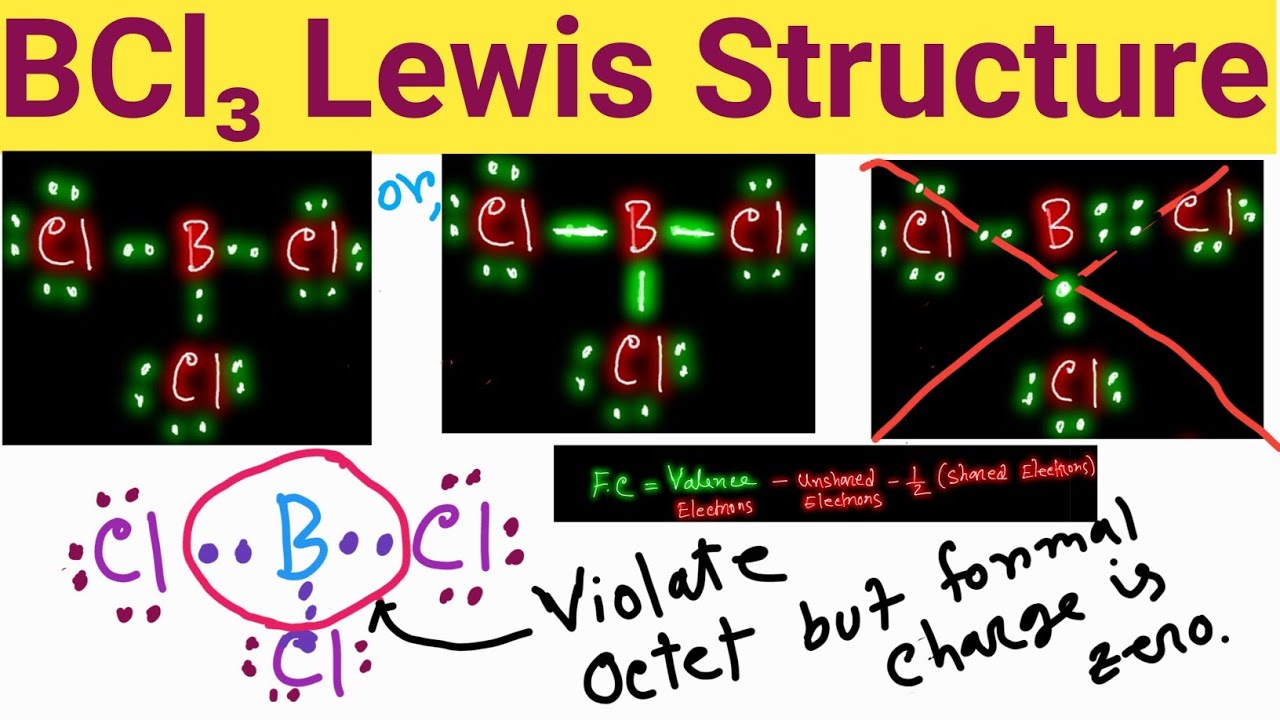
The BCl3 Lewis structure represents the arrangement of electrons around the boron atom in the center, bonded to three chlorine atoms. Boron has three valence electrons, while each chlorine atom contributes seven valence electrons, leading to a total of 24 valence electrons available for bonding in the molecule. This structure can be depicted using dots to represent electrons and lines to signify bonds.
How to Draw the BCl3 Lewis Structure?
Drawing the BCl3 Lewis structure involves a systematic approach:
- Count the total number of valence electrons available.
- Identify the central atom (boron) and arrange the surrounding atoms (chlorine).
- Distribute the electrons to form bonds between boron and the chlorine atoms.
- Ensure that each atom achieves a stable electron configuration, typically an octet for chlorine and three electrons for boron.
What is the Molecular Geometry of BCl3?
The molecular geometry of BCl3 is trigonal planar. This arrangement occurs due to the three bonding pairs of electrons around the boron atom, which repel each other and spread out in a planar configuration. The bond angles in BCl3 are approximately 120 degrees, reflecting the symmetrical distribution of the chlorine atoms around the boron center.
What Are the Properties of BCl3?
Boron trichloride possesses several noteworthy properties that make it an interesting compound in the realm of chemistry:
- It is a colorless gas at room temperature.
- BCl3 has a pungent odor and is corrosive to the skin and eyes.
- It reacts vigorously with water, releasing hydrochloric acid.
- This compound is used in various chemical syntheses and as a reagent in organic chemistry.
What Are the Applications of BCl3?
Boron trichloride is utilized in several applications, including:
- Synthesis of organoboron compounds.
- As a catalyst in chemical reactions.
- In the semiconductor industry for doping processes.
- As a reagent in the production of pharmaceuticals.
What Are the Safety Precautions for Handling BCl3?
Due to its hazardous nature, specific safety precautions should be taken when handling BCl3:
- Always use personal protective equipment (PPE), including gloves, goggles, and lab coats.
- Handle the compound in a well-ventilated area or fume hood.
- Be aware of the appropriate first aid measures in case of exposure.
- Store BCl3 in a cool, dry place away from moisture and incompatible substances.
How Does the BCl3 Lewis Structure Influence Its Reactivity?
The BCl3 Lewis structure sheds light on the compound's reactivity. The electron-deficient nature of boron makes it a Lewis acid, enabling it to accept electron pairs from other molecules. This characteristic is significant in various chemical reactions, particularly in organic synthesis, where it can facilitate bond formation and rearrangement.
Conclusion: Importance of Understanding the BCl3 Lewis Structure
In summary, the BCl3 Lewis structure is a valuable tool for predicting the properties and reactivity of boron trichloride. By understanding its molecular geometry, bonding, and electron arrangement, chemists can better utilize this compound in diverse applications. Whether you are a student, a researcher, or a chemistry enthusiast, grasping the concepts surrounding the BCl3 Lewis structure will undoubtedly enhance your comprehension of chemical bonding and molecular interactions.
Article Recommendations
- Shawn Pomrenke Net Worth 2023 Estimate Details
- Mitch Mcconnells Blocked Legislation The Extent Of Obstruction
- About Sami Yusufs Wife Who Is She